EGRIFTA WRTM Dosing and Administration1,2
Once-weekly reconstitution for simpler daily administration
Once-weekly reconstitution for simpler daily administration

Once-daily dosing of 1.28 mg
Concentrated for small daily injection volumes (0.16 mL)
Stored at room temperature (no refrigeration required before or after reconstitution)
Comes with BD SafetyGlideTM syringes for injection (with needle attached) (orange cap)
EGRIFTA WRTM was designed for simpler daily administration, thereby reducing the burden of treatment.

| Medication Box | |
|---|---|
| Quantity | Item |
| 4 | Single-patient use EGRIFTA WRTM 11.6 mg vials (1 vial per week for weekly mixing) |
| Injection Box | |
|---|---|
| Quantity | Item |
| 1 | 30 mL bottle of Bacteriostatic Water for Injection (for weekly mixing) |
| 4 | Sterile BD Plastipak™ 3 mL syringes with needle attached (clear cap), for weekly mixing |
| 33 | Sterile BD SafetyGlide™ syringes with needle attached (orange cap), for daily injection |
| 1 | Box of 100 alcohol swabs |
Each box contains supplies for 28 days of treatment.
One EGRIFTA WRTM 11.6 mg vial must be reconstituted weekly with 1.3 mL of Bacteriostatic Water for Injection to prepare seven daily injection doses.
The daily dose of EGRIFTA WRTM is 1.28 mg (0.16 mL of the reconstituted solution), administered once daily. Each vial provides seven consecutive daily injections.
Patients will need:
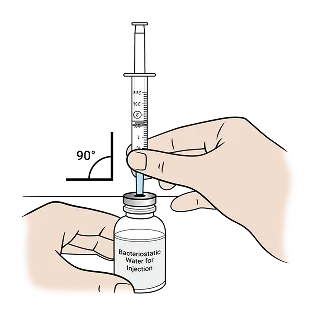
Using the BD PlastipakTM 3 mL syringe (clear cap), draw 1.5 mL of Bacteriostatic Water for Injection from the bottle. This syringe is dedicated to weekly mixing.
Push the plunger up slowly to remove 0.2 mL of excess water and air out of the syringe and align the top rim of the plunger to the 1.3 mL mark.
The Bacteriostatic Water for Injection bottle should be placed back in the Injection Box for the next weekly mixing.
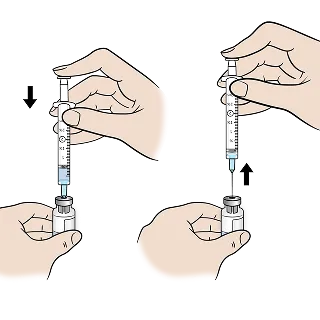
Insert the needle into the EGRIFTA WRTM vial, then push the plunger down to empty the syringe, allowing water to mix with the powder.
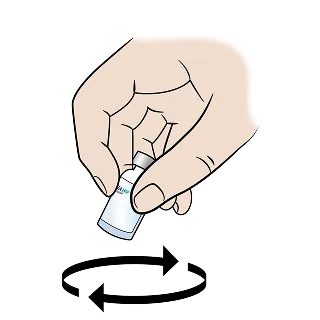
Instruct patients not to shake the vial. Instead, gently swirl the vial until the powder fully dissolves, resulting in a clear, colorless solution.
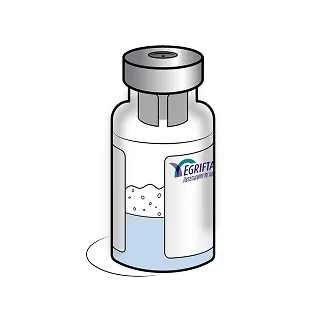
If the solution remains cloudy, discolored, or contains particles, patients should discard the vial and contact their healthcare provider or THERA patient support®.
Patients will need:
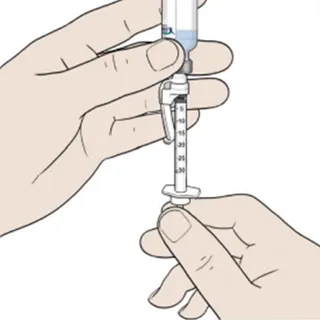
Insert the BD SafetyGlideTM syringe (orange cap) into the vial and pull the plunger to the 0.18 mL mark. The daily dose is withdrawn from the same vial for 7 consecutive days.
Gently tap the syringe to force any air bubbles to rise to the top.
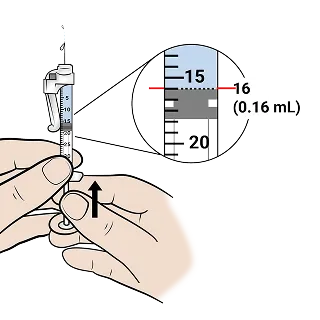
Slowly push the plunger up to remove air and excess liquid until 0.16 mL mark is reached.
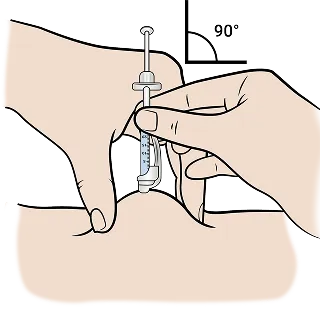
The recommended injection site is the abdomen, avoiding scar tissue, bruises, hard bumps from previous injections, the belly button, or areas within 2 inches of the belly button.
Advise patients to rotate the site for each injection.
This video was designed to be a step-by-step guide to help your patients learn how to mix and inject EGRIFTA WRTM.

For complete instructions, read the full Instructions for Use
DownloadAccess dosing and administration resources for you and your patients.

Provide your patients with step-by-step instructions on how to mix and inject EGRIFTA WRTM.

A detailed video walkthrough for preparing and administering EGRIFTA WRTM will be available shortly. Please check back soon for updates.

Explore THERA patient support® program resources.
Enter your contact details to stay up to date with the latest developments from Theratechnologies.
EGRIFTA WRTM is indicated for the reduction of excess abdominal fat in HIV-infected adult patients with lipodystrophy.
Do not use EGRIFTA WRTM if patient:
The most commonly reported adverse reactions include injection site reactions, arthralgia, pain in extremity, myalgia, and peripheral edema.
For complete disclosure of EGRIFTA WRTM product information, please read the Full Prescribing Information, Patient Information, and Patient Instructions for Use.
For more information about EGRIFTA WRTM, contact 
