Subcutaneous Fat
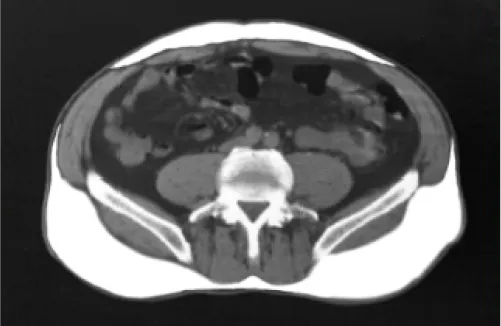
CT scan of abdomen at L4
BMI
In typical obesity (BMI >30), weight is carried throughout the body and fat accumulates primarily as subcutaneous adipose tissue (SAT).8,9
Location
Just beneath the skin8,9
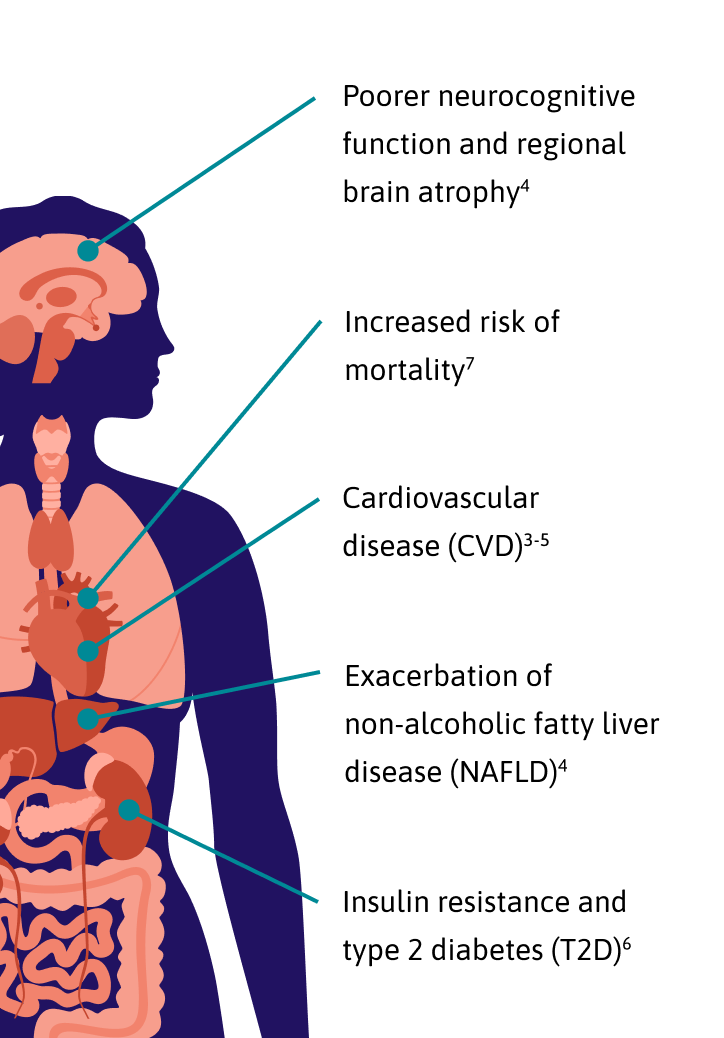
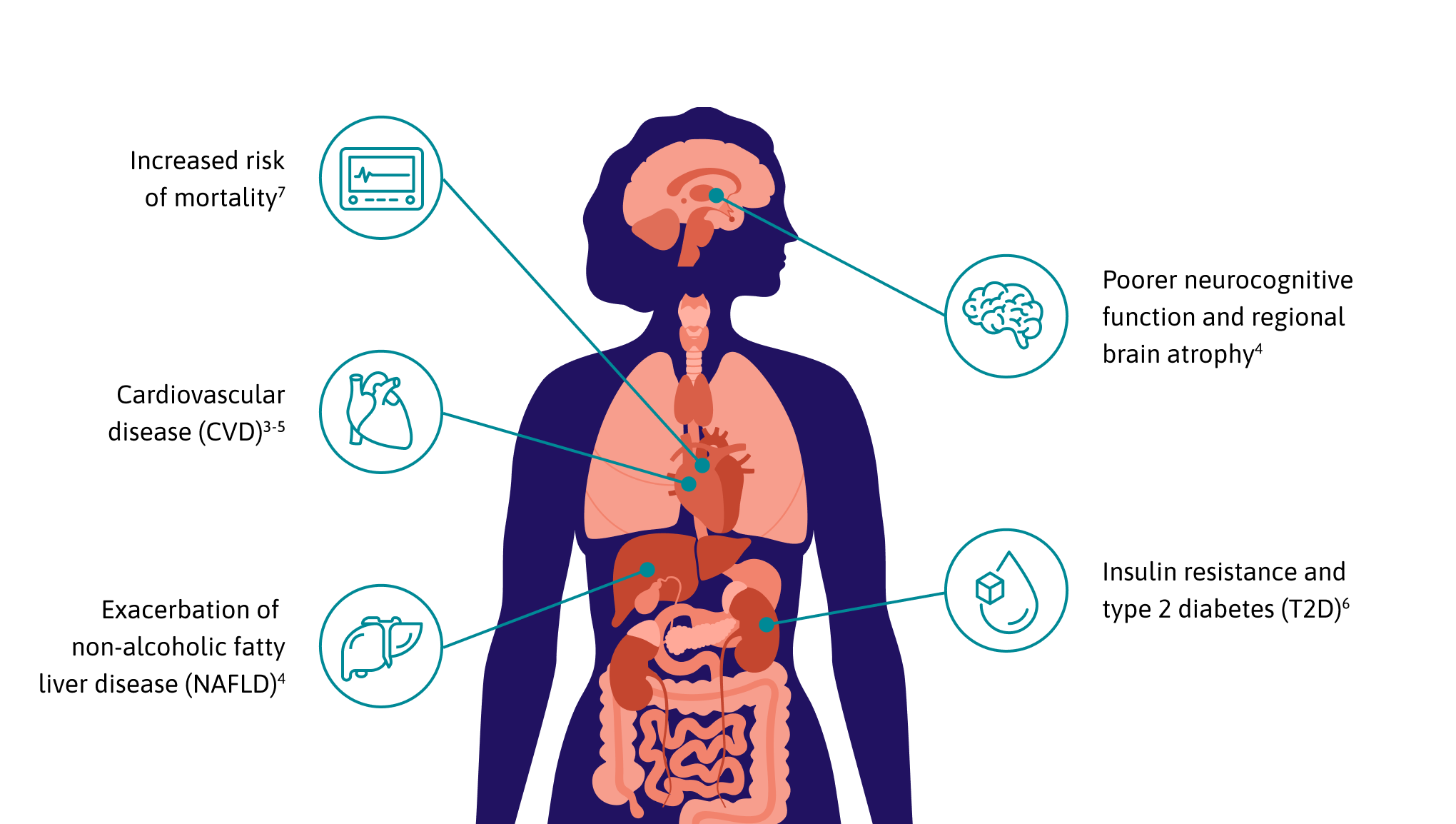
With advancements in antiretroviral therapy (ART), PWH now enjoy the same life expectancy as those without the virus, making long-term health management a priority.
However, even in the era of modern ART, studies show that the condition of central adiposity remains a common side effect.1
Central adiposity refers to the localized accumulation of both subcutaneous and visceral adipose tissue (VAT) in and around the abdomen. In PWH, this is often characterized by EVAF.
EVAF is an abnormal buildup of VAT within the abdominal cavity surrounding the internal organs.2

Studies show that up to 60% of PWH on modern ART with virological suppression for over a year were found to have EVAF.3*
* Based on a multi-center observational study in PWH.3
EGRIFTA WRTM is not approved for use in clinical conditions other than the reduction of EVAF.
Recent findings also indicate that a larger VAT surface area is linked to a significantly higher 10-year ASCVD risk.3†

CT scan of abdomen at L4
In typical obesity (BMI >30), weight is carried throughout the body and fat accumulates primarily as subcutaneous adipose tissue (SAT).8,9
Just beneath the skin8,9
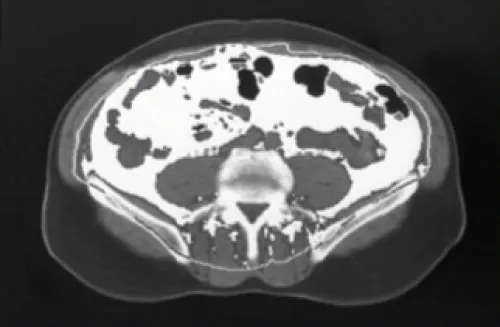
In PWH, weight gain can occur irrespective of a high BMI and is characterized by the buildup of VAT.2,8
Visceral fat surface area in an actual patient. Surface area above 130 cm2 is indicative of lipohypertrophy.8
* Based on a multi-center observational study in PWH.10
CT scan of abdomen at L4
Poor indicator of EVAF as it is prevalent even in PWH with normal BMI, with rates of:10*
Explore steps to easily and accurately identify EVAF
Learn MoreDeep within the abdominal cavity, surrounding the internal organs2,8
Surface area: 155 cm2*
While the exact causes behind developing EVAF remain unknown, there are several factors that can put the patient at increased risk, including:
HIV virus4,11
Duration of ART4,11
Chronic inflammation12
Age: >40 years old11
Assigned female at birth11
Altered growth hormone (GH) secretion3
Recent data highlight a strong correlation between disturbances in GH levels and VAT in PWH.3
Measure waist circumference (WC)2
WC ≥37.4 in (95 cm)
WC ≥37 in (94 cm)
Studies show that WC measurement is the most reliable predictor of EVAF, outperforming BMI.13
If WC meets the threshold for EVAF, proceed to measure hip circumference and calculate the waist-to-hip ratio (WHR).
Ensure both circumferences are entered with the same unit.
WHR ≥0.94
WHR ≥0.88

Equip your patients with an informed approach to discussing EVAF and treatment with EGRIFTA WRTM
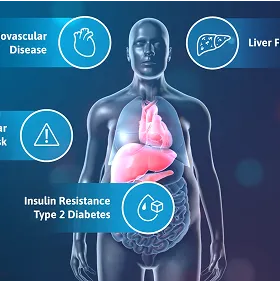
Explore how EGRIFTA WRTM helps address the reduction in GH concentrations caused by EVAF
† Waist-to-hip ratio = waist circumference/hip circumference.
‡ Based on the Visceral Adiposity Measurement and Observations Study (VAMOS): a cross-sectional, multi-center observational study in PWH with ART-induced virological suppression for ≥1 year and a BMI range of 20 to 40 kg/m2.
§ Reference values are based on inclusion criteria in clinical trials.
ART = antiretroviral therapy; ASCVD = atherosclerotic cardiovascular disease; BMI = body mass index; CVD = cardiovascular disease; EVAF = excess visceral abdominal fat; GH = growth hormone; HC = hip circumference; MOA = mechanism of action; PWH = people with HIV; SAT = subcutaneous adipose tissue; VAT, visceral adipose tissue; WC = waist circumference; WHR = waist-to-hip ratio.
Enter your contact details to stay up to date with the latest developments from Theratechnologies.
EGRIFTA WRTM is indicated for the reduction of excess abdominal fat in HIV-infected adult patients with lipodystrophy.
Do not use EGRIFTA WRTM if patient:
The most commonly reported adverse reactions include injection site reactions, arthralgia, pain in extremity, myalgia, and peripheral edema.
For complete disclosure of EGRIFTA WRTM product information, please read the Full Prescribing Information, Patient Information, and Patient Instructions for Use.
For more information about EGRIFTA WRTM, contact 
