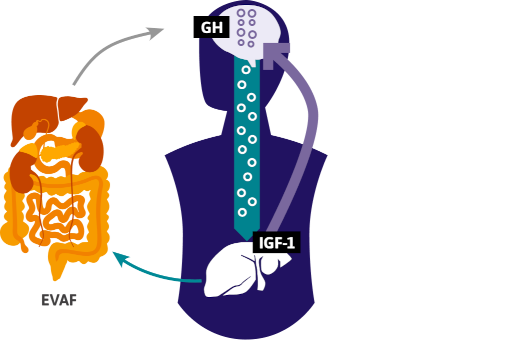
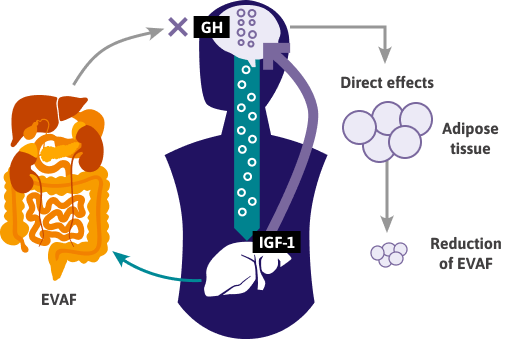
- GH is essential in the formation and function of fat cells and plays a key role in the overall regulation of fat metabolism.4
- VAT accumulation is predictive of low GH levels; in PWH, there is a significant inverse relationship between increasing VAT surface area and lower GH levels.1
- EVAF may directly reduce GH levels, while diminished GH and elevated IGF-1 levels further drive EVAF buildup, creating a maladaptive feedback loop.2


 toll-free at 1-833-23THERA (1-833-238-4372). To report suspected adverse reactions, contact
toll-free at 1-833-23THERA (1-833-238-4372). To report suspected adverse reactions, contact